Which of the Following Statements About Subatomic Particles Is True
O Neutrons and electrons are found in the nucleus of an atom. Electrons and protons are located within the nucleus.
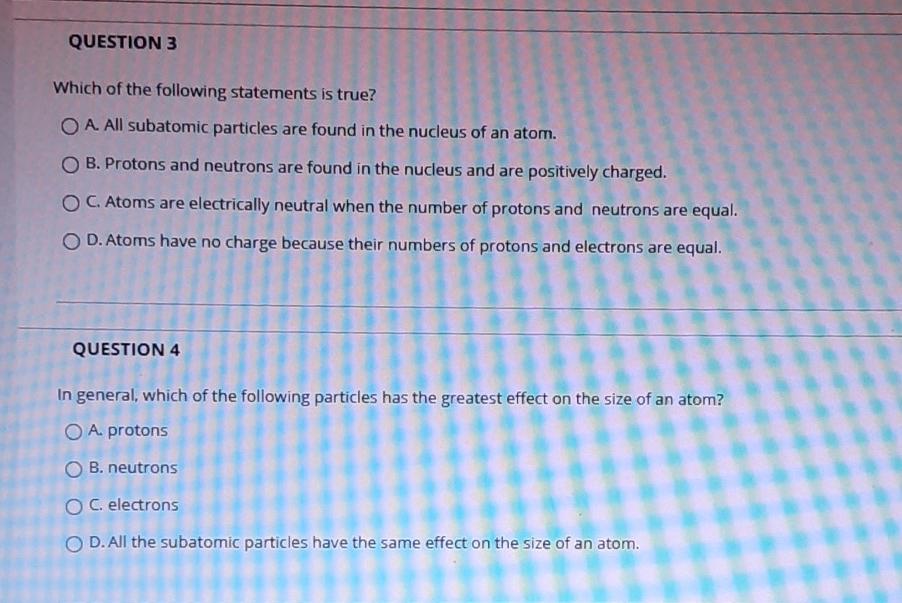
Solved Question 3 Which Of The Following Statements Is True Chegg Com
Is it true that according to quantum mechanics the motions of subatomic particles may be described as wavelike.
. Neutron has a mass of 16741 x10-27 kg. Think about which type of peer pressure the statements fall into. Select ALL that apply the particles will have the same kinetic energy the space between the particles is different with.
The statement which is true about sub atomic particles is electrons are the subatomic particles with the smallest mass. This is a true statement because opposite charges attract each other whereas like charges repel each other. Electrons are the subatomic particles with the smallest mass since it has a mass of 91094 x 10 31 kg while protons has a mass of 16726 x 10-27 kg.
23 23 Which of the following statements about subatomic particles is TRUE. O Neutrons and electrons are found in the nucleus of an atom. Protons are positively charged and the lightest subatomic particle.
O Protons and neutrons have the same mass. Which statement about subatomic particles is true. The mass of an atom is concentrated at the nucleus.
Electrons have very little mass compared to protons and neutrons. Proton has approximately the same mass with neutron. Select all that apply A.
Osmium one of the densest elements on earth has an actual mass of 19023 grams. Electrons are negatively charged and are the heaviest subatomic particle. The charge of electron is opposite to the charge of proton.
Which subatomic particle plays the greatest part in determining the properties of an element. O Electrons make up most of the mass of an atom. Electrons and protons are located within the nucleus.
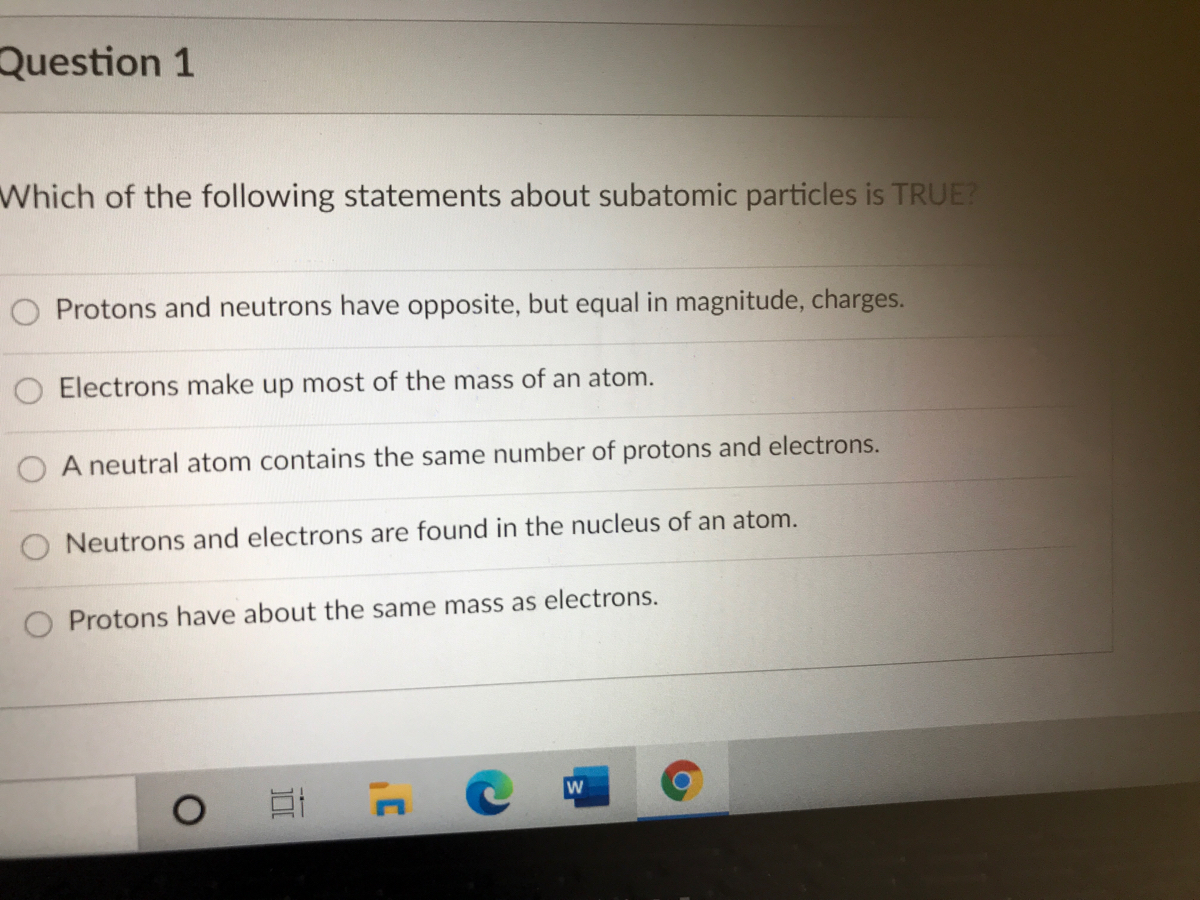
Which of the following statements about subatomic particles is TRUE. O A neutral atom contains the same number of protons and electrons. O Protons weigh less than electrons.
O Protons weigh less than electrons. According to the table above what is its value in terms of atomic mass units. Correct answer - Which of the following statements about subatomic particles is TRUE.
Which of the following statements about subatomic particles is TRUE A A neutral from CHEM 101 at Drexel University. Atoms is known to have three basic particles which are protons electrons and neutrons. Protons and neutrons have opposite but equal in magnitude charges.
Label each type as put down reasoning rejection or Acid precipitation lowered the ph of soil in a terrestrial ecosystem that supported a diverse community of plants and animals. There are subatomic particles the electron is known to have the smallest mass. Electrons are the subatomic particles with the smallest mass.
The charge on a proton is equal in magnitude and opposite in sign to that of an electron C. I II and IV C. B Protons and neutrons have opposite but equal in magnitude charges.
B A neutral atom contains the same number of protons and electrons. Which statement about subatomic particles is true. Select ALL that apply the particles will have the same kinetic energy the space between the particles is different with.
This is a true statement because the charge of the proton and the electron are equal in magnitude but opposite in sign so that when the two particles are paired the charges sum to zero. Protons are the only subatomic particles to have charge. A neutral atom contains the same number of protons and electrons.
Of 11840 of an atomic mass unit amu. Which statement is true about the particles in liquid water at 100ºC and the particles in steam at 100ºC. O Electrons make up most of the mass of an atom.
O Protons have about the same mass as electrons. Which of the following statements about the masses of subatomic particles is true. Which statement is true about the particles in liquid water at 100ºC and the particles in steam at 100ºC.
Which of the following is true about subatomic particles. Proton has approximately the same mass with neutron. A Protons neutrons and electrons are the three subatomic particles present in an atom.
Protons and neutrons are present inside the nucleus of atom and electrons are present outside the nucleus. Which of the following statements is true about the subatomic particles of an atom. Which subatomic particle is electrically neutral and found in the nucleus of an atom.
Which of the following statements about subatomic particles is true AnswerProtons have about the same mass as electrons is falseExplanationElectrons and protons have the same magnitude on charge but opposite sign. The charge of electron is opposite to the charge of proton. Which of the following statements about subatomic particles is TRUE.
I III and IV D. The mass of a proton is similar to that of a neutron D. Neutrons have no charge and are the lightest subatomic particle.
The 3 subatomic particles share key differences and similarities in their masses and charges. A A neutral atom contains the same number of protons and electrons. It is a nucleon.
Protons neutrons and electrons all have about the same mass. C Electrons make up most of the mass of an atom. Quantum Mechanics describes subatomic particles as particles even light is.
Protons weigh less than neutrons. Charge of proton Neutrons have no chargeAn atom is composed. III and III B.
Which of the following statements are TRUE about the subatomic particles. Which statement about subatomic particles is true. D Protons have about the same mass as electrons.
B For a neutral atom the atomic number is equal to the proton number. I II and III B. Which of the following statements about subatomic particles are correct.
Neutrons and protons are the only charges subatomic particles B. A protons weigh more than electrons and neutrons b protons weigh Subjects. The mass of a neutron nearly equals the mass of a proton.
Charge of electron. Subatomic particles all have the same mass. The true statement about subatomic particles is that Electrons are the subatomic particles with the smallest mass.
Neutrons orbit the nucleus of the atom. The mass of an atom is concentrated at the nucleus. Which of the following statements about subatomic particles are true and which are false.
The following statement describes which subatomic particle best.

Answered Which Of The Following Statements About Bartleby
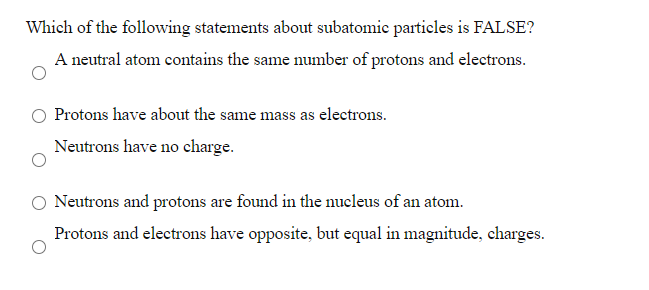
Solved Which Of The Following Statements About Subatomic Chegg Com

Solved Which Of The Following Statements About Subatomic Chegg Com
Comments
Post a Comment